Sakracy
Product description/product information
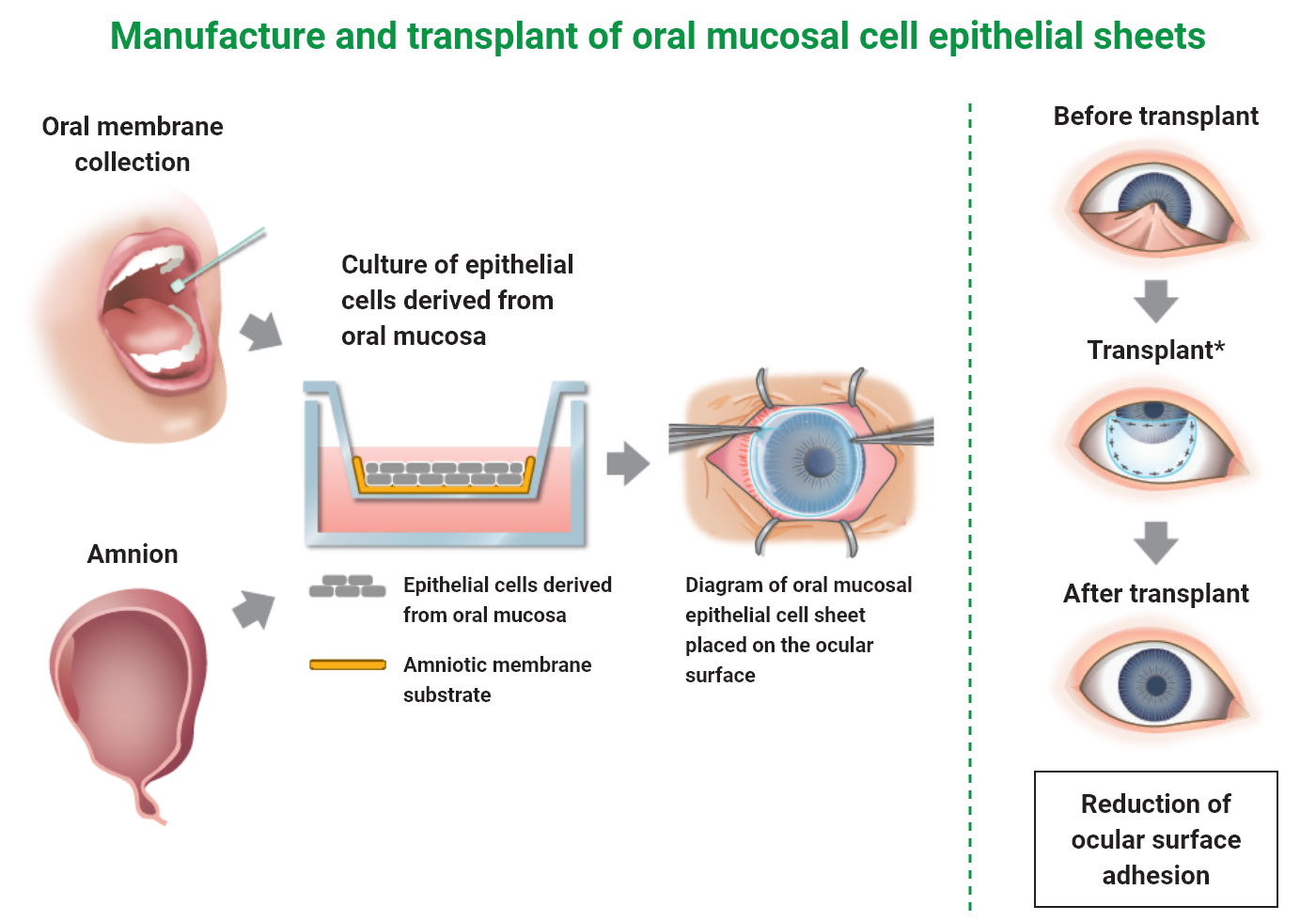
Sakracy is a cultured autologous oral mucosal epithelial cell sheet consisted with patient’s own oral mucosal epithelial cells, which are isolated from the patient’s tissue and then seeded and cultured on an amniotic membrane substrate prepared from human allogeneic amniotic membrane. Sakracy is transplanted onto the exposed cornea or sclera of a patient with ocular surface diseases accompanying limbal stem cell deficiency, such as adhesion and conjunctival fornix shortening, after adhesion release or scar tissue removal so that the oral mucosal epithelial cells will be engrafted and epithelialized, leading to the repair of any ocular surface abnormality.
-
Brand name
-
Product name: Sakracy
Nonproprietary name: Human (autologous) oral mucosa-derived epithelial cell sheet using human amniotic membrane substrate
Brand name: Sakracy
-
Marketing Authorization Holder
-
Hirosaki Lifescience Innovation, Inc.
-
JAN code
-
Oral mucosal tissue transport set: 4595124264026
Culture autologous oral mucosal epithelial cell sheet package: 4595124264019
-
Approval No.
-
30400FZX00001000
-
Date of approval
-
January 2022
-
Reimbursement listing
-
September 2022
Indication or performance
Alleviation of adhesion on the ocular surface accompanying limbal stem cell deficiency
Related precautions
- As Sakracy is not intended to be used for the treatment of etiologies of limbal stem cell deficiency, it should be used after the management of causative etiologies of limbal stem cell deficiency.
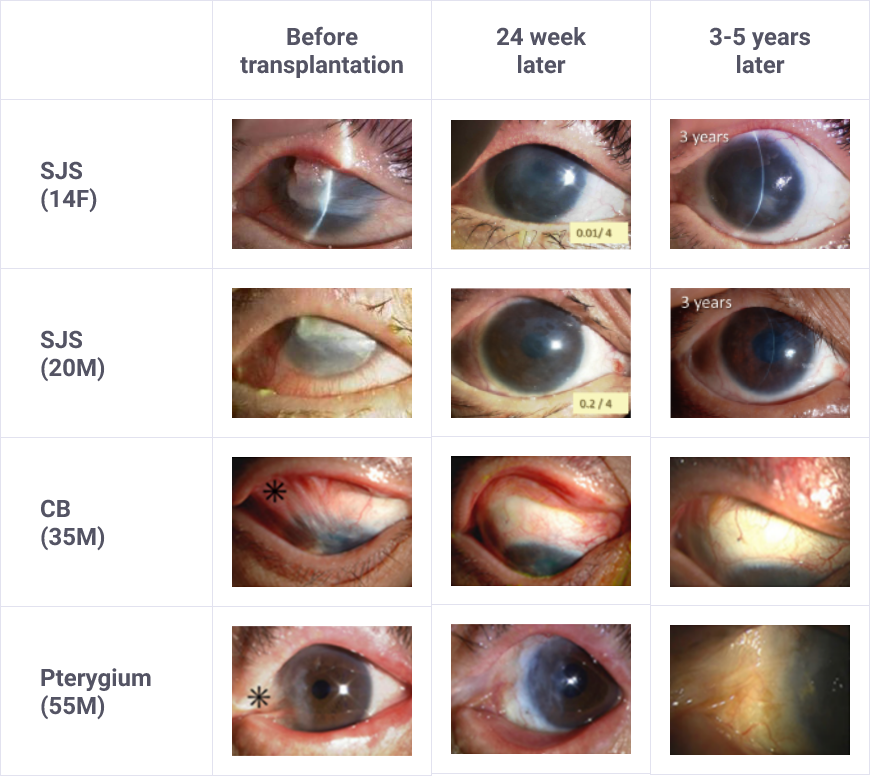
- Eligible patients should be selected with a full understanding of the description of clinical studies for the characteristics (e.g., degree of adhesion) of patients included in clinical studies and of the efficacy and safety of Sakracy.
Product composition and dosage form
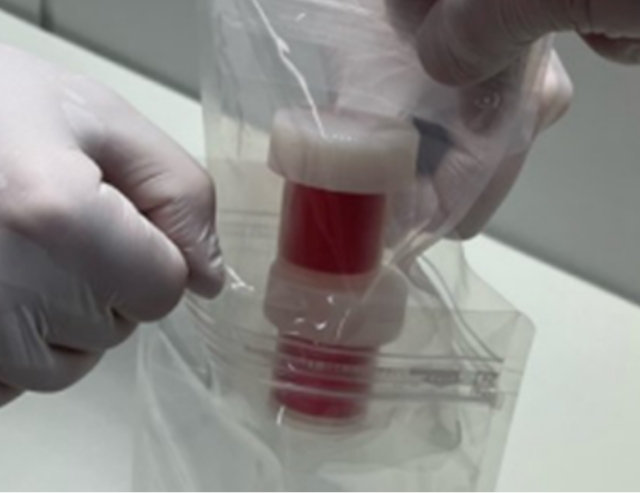
Oral mucosal tissue transport set

Cultured autologous oral mucosal epithelial cell sheet package
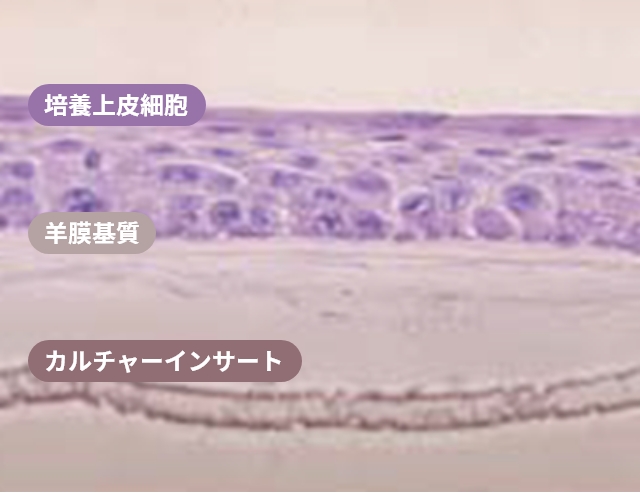
H&E staining image of multiple layers of oral mucosal epithelial cells on an amniotic membrane substrate
Ref. Nakamura, T et al. Br J Opthalmol 2004
History of Development

Dr. Shigeru Kinoshita
Emeritus Professor, Kyoto Prefectural University of Medicine
Professor, Medical Science and Technology for Ophthalmology, Kyoto Prefectural University of Medicine (since 2015)
Honorary member, Japan Cornea Society
Director, Keratoplasty Society of Japan (FY2023)

Dr. Chie Sotozono
Professor, Department of Ophthalmology, Kyoto Prefectural University of Medicine (since 2015)
Executive Director, Japanese Ophthalmological Society (from 2023 to 2025)
Director, Japan Cornea Society (from 2023 to 2025)
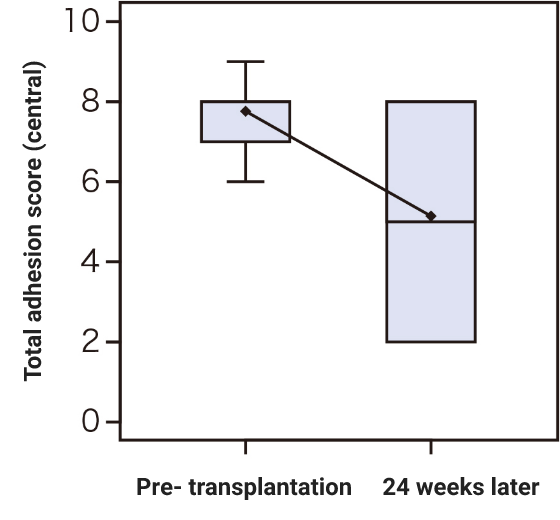
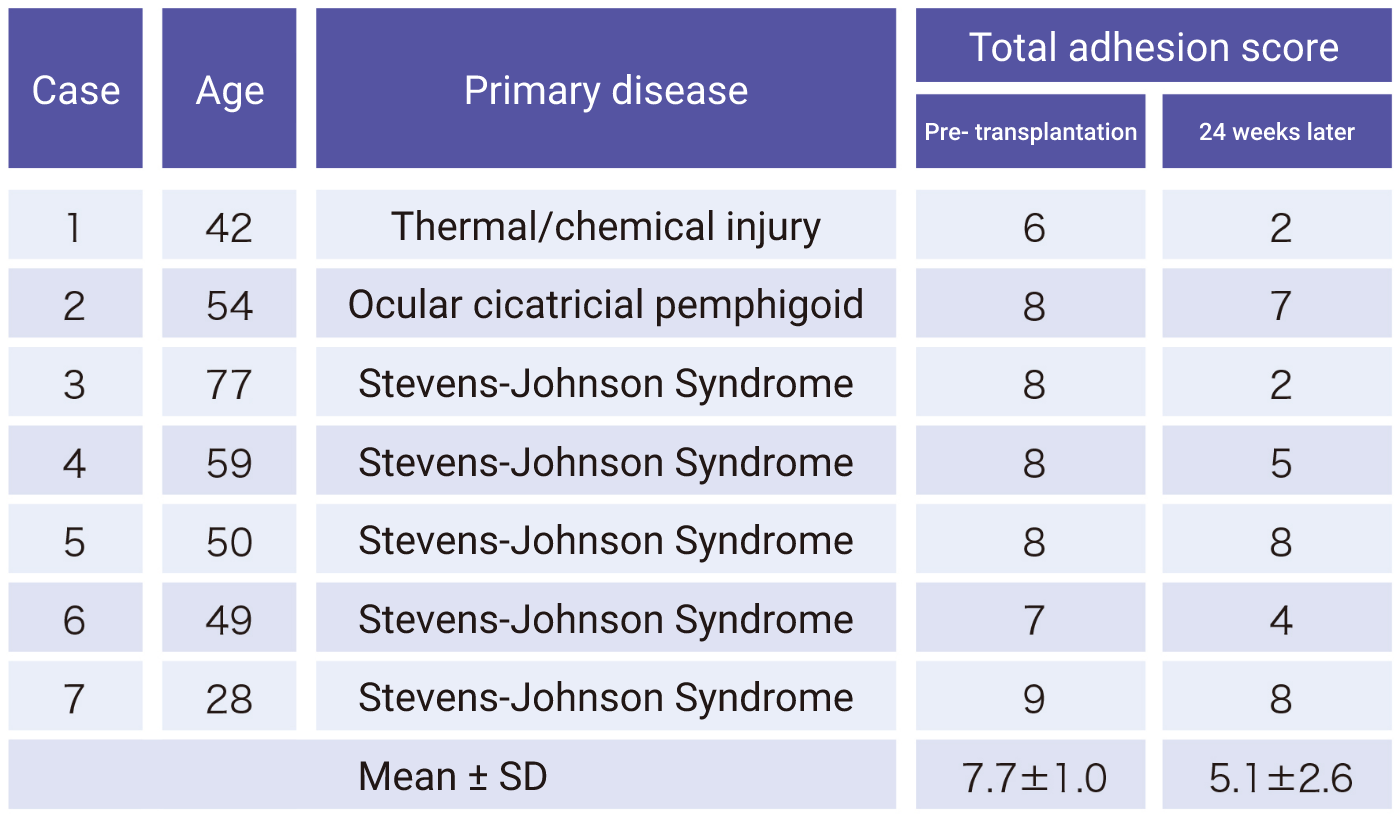
Research on cultured autologous oral mucosal epithelial cell sheets has been conducted by Dr. Shigeru Kinoshita and colleagues from the Department of Ophthalmology, Kyoto Prefectural University of Medicine. A clinical investigation was started in 2002, and a total of 72 patients underwent transplantation as of 2008. A retrospective analysis of these patients confirmed the efficacy of cultured autologous oral mucosal epithelial cell sheets.
Subsequently, a clinical investigation under the Advanced Medical Care B program (from July 2014 to September 2017) supported by Health, Labour, and Welfare Sciences Research Grants was conducted, and an investigator-initiated trial in subjects with severe stem cell deficiencies for the release of adhesions on the ocular surface titled “A multicenter, single-arm, phase 3 study of transplantation of cultured autologous oral mucosal epithelial sheets to supply mucosal epithelium in patients with refractory ocular surface diseases” (from October 2018 to September 2019) was conducted by Dr. Chie Sotozono, Department of Ophthalmology, Kyoto Prefectural University of Medicine, with support for the production by FBRI.
To enable the provision of a new treatment option for patients with severe limbal stem cell deficiencies, our company submitted a marketing authorization application to the Ministry of Health, Labour and Welfare based on the results from the investigator-initiated trial and received approval for Sakracy on January 20, 2022, with the product beginning to be covered by insurance on September 1, 2022.
“Sakura” (cherry blossoms) is a common symbol between Hirosaki City, in which our company is founded, and Kyoto City, which is the development base for the product. This product was named “Sakracy” in the hope that patients will experience visual acuity improvement and can view and enjoy sakura (cherry blossoms).
Department of Ophthalmology, Kyoto Prefectural University of MedicineTarget patients
Schemes
Clinical trial